Aristolochene synthase
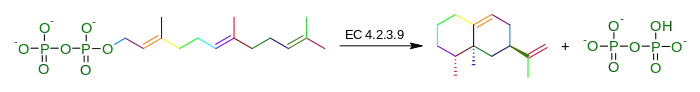
In enzymology, an aristolochene synthase (EC 4.2.3.9) is an enzyme that catalyzes the chemical reaction
| aristolochene synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.2.3.9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Hence, this enzyme has one substrate, 2-trans,6-trans-farnesyl diphosphate, and two products, aristolochene and diphosphate.
This enzyme belongs to the family of lyases, specifically those carbon-oxygen lyases acting on phosphates. The systematic name of this enzyme class is 2-trans,6-trans-farnesyl-diphosphate diphosphate-lyase (cyclizing, aristolochene-forming). Other names in common use include sesquiterpene cyclase, trans,trans-farnesyl diphosphate aristolochene-lyase, trans,trans-farnesyl-diphosphate diphosphate-lyase (cyclizing,, and aristolochene-forming). This enzyme participates in terpenoid biosynthesis.
This protein may use the morpheein model of allosteric regulation.[1]
Structural studies
As of late 2007, two structures have been solved for this class of enzymes, with PDB accession codes 2E4O and 2OA6. They are both notable for the very high helix content of the structure.
References
- T. Selwood & E. K. Jaffe (2011). "Dynamic dissociating homo-oligomers and the control of protein function". Arch. Biochem. Biophys. 519 (2): 131–43. doi:10.1016/j.abb.2011.11.020. PMC 3298769. PMID 22182754.
- Cane DE, Prabhakaran PC, Oliver JS, McIlwaine DB (1990). "Aristolochene biosynthesis. Stereochemistry of the deprotonation steps in the enzymatic cyclization of farnesyl pyrophosphate". J. Am. Chem. Soc. 112 (8): 3209–3210. doi:10.1021/ja00164a051.
- Noguchi H, Rawlings BJ (1989). "Aristolochene biosynthesis and enzymatic cyclization of farnesyl pyrophosphate". J. Am. Chem. Soc. 111 (24): 8914–8916. doi:10.1021/ja00206a022.
- Hohn TM, Plattner RD (1989). "Purification and characterization of the sesquiterpene cyclase aristolochene synthase from Penicillium roqueforti". Arch. Biochem. Biophys. 272 (1): 137–43. doi:10.1016/0003-9861(89)90204-X. PMID 2544140.
- Proctor RH, Hohn TM (1993). "Aristolochene synthase. Isolation, characterization, and bacterial expression of a sesquiterpenoid biosynthetic gene (Ari1) from Penicillium roqueforti". J. Biol. Chem. 268 (6): 4543–8. PMID 8440737.
- Calvert MJ, Ashton PR, Allemann RK (2002). "Germacrene A is a product of the aristolochene synthase-mediated conversion of farnesylpyrophosphate to aristolochene". J. Am. Chem. Soc. 124 (39): 11636–41. doi:10.1021/ja020762p. PMID 12296728.