Uroporphyrinogen-III C-methyltransferase
Uroporphyrinogen-III C-methyltransferase (EC 2.1.1.107), uroporphyrinogen methyltransferase, uroporphyrinogen-III methyltransferase, adenosylmethionine-uroporphyrinogen III methyltransferase, S-adenosyl-L-methionine-dependent uroporphyrinogen III methylase, uroporphyrinogen-III methylase, SirA, CysG, CobA, uroporphyrin-III C-methyltransferase, S-adenosyl-L-methionine:uroporphyrin-III C-methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:uroporphyrinogen-III C-methyltransferase.[1][2][3] This enzyme catalyses the following chemical reaction
- 2 S-adenosyl-L-methionine + uroporphyrinogen III 2 S-adenosyl-L-homocysteine + precorrin-2 (overall reaction)
- (1a) S-adenosyl-L-methionine + uroporphyrinogen III S-adenosyl-L-homocysteine + precorrin-1
- (1b) S-adenosyl-L-methionine + precorrin-1 S-adenosyl-L-homocysteine + precorrin-2
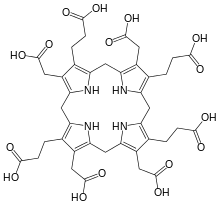 uroporphyrinogen III substrate of the enzyme |
 precorrin-2 product of the enzyme |
| Uroporphyrinogen-III C-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.1.1.107 | ||||||||
| CAS number | 125752-76-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Uroporphyrinogen-III C-methyltransferase catalyses two methylation reactions. The first reaction converts uroporphyrinogen III into precorrin-1. The second converts precorrin-1 into precorrin-2. These reactions are part of the biosynthetic pathway to cobalamin (vitamin B12) in both anaerobic and aerobic bacteria.
See also
References
- Warren MJ, Gonzalez MD, Williams HJ, Stolowich NJ, Scott AI (1990). "Uroporphyrinogen-III methylase catalyzes the enzymatic-synthesis of sirohydrochlorin-II and sirohydrochlorin-IV by a clockwise mechanism". J. Am. Chem. Soc. 112: 5343–5345. doi:10.1021/ja00169a048.
- Warren MJ, Roessner CA, Santander PJ, Scott AI (February 1990). "The Escherichia coli cysG gene encodes S-adenosylmethionine-dependent uroporphyrinogen III methylase". The Biochemical Journal. 265 (3): 725–9. doi:10.1042/bj2650725. PMC 1133693. PMID 2407234.
- Schubert HL, Raux E, Brindley AA, Leech HK, Wilson KS, Hill CP, Warren MJ (May 2002). "The structure of Saccharomyces cerevisiae Met8p, a bifunctional dehydrogenase and ferrochelatase". The EMBO Journal. 21 (9): 2068–75. doi:10.1093/emboj/21.9.2068. PMC 125995. PMID 11980703.
External links
- Uroporphyrinogen-III+C-methyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)